Share Article
Improvement in liver histology demonstrated in 53 percent of patients
Foster City, CA -- June 22, 2001
Gilead Sciences, Inc. (Nasdaq: GILD) today announced interim 48-week data from a Phase III clinical trial (Study 437) evaluating the safety and efficacy of adefovir dipivoxil once daily as monotherapy compared to placebo in patients with chronic hepatitis B virus (HBV) infection. Results from this intent-to-treat analysis meet the study's primary endpoint of improvement in liver histology at week 48 compared to baseline. Improvement in liver histology was observed in 53 percent of patients treated with adefovir dipivoxil 10 mg, compared to 25 percent of placebo-treated patients, as measured by liver biopsies (p<0.001). change="" in="" liver="" histology="" is="" an="" important="" marker="" of="" disease="" progression="" in="" patients="" with="" chronic="" hbv="" infection.="" gilead="" anticipates="" the="" presentation="" of="" the="" complete="" analyses="" of="" study="" 437="" at="" scientific="" conferences="" later="" this="" year="" and="" in="" 2002.="">
"A therapy's ability to improve liver histology is among the best clinical markers to measure activity against hepatitis B," said Patrick Marcellin, MD, Service d'Hepatologie, Hôpital Beaujon, Clichy and Professor, Université Paris VII, Paris, France. "The data on liver histology in this Phase III study are encouraging and highlight the potential of adefovir dipivoxil to become an important therapy for the many patients with chronic hepatitis B infection. Additionally, resistance is emerging as a significant issue for physicians and their patients, and the lack of resistance development to adefovir dipivoxil after one year of therapy observed in this study is promising."
Study 437 Design
Study 437 is a two-year, randomized, double-blind, placebo-controlled Phase III trial designed to evaluate the safety and efficacy of adefovir dipivoxil given once daily, compared to placebo. In this study, two doses of adefovir dipivoxil were evaluated, including the 10 mg dose for which Gilead will seek marketing approval and an exploratory 30 mg dose for the first 48 weeks. This study enrolled 515 patients in the United States, Canada, Europe, Australia and Southeast Asia. During the first year of Study 437, 172 patients were randomized to the adefovir dipivoxil 10 mg arm, 173 to the adefovir dipivoxil 30 mg arm and 170 to placebo.
At baseline, patients had a median serum HBV DNA of 8.36 log10 copies/mL and a median ALT (elevated blood levels of enzyme markers that indicate liver disease) of 94 IU/L. At study entry, a majority of patients had not received prior treatment for their HBV infection. The effect of one year of treatment with adefovir dipivoxil 10 mg compared to placebo was evaluated in terms of improvements in liver histology, rates of seroconversion, changes in HBV viral load and other important markers of liver disease. Seroconversion is defined as both the disappearance of the hepatitis B "e" antigen (HBe-antigen negative), a marker of HBV replication, and the appearance of antibodies specific for this antigen (HBe-antibody positive). During the second year of Study 437, researchers will continue to evaluate the long-term efficacy, safety and resistance profile of adefovir dipivoxil.
Efficacy Results
In addition to improvement in liver histology, seroconversion was observed in 12 percent of patients treated with adefovir dipivoxil 10 mg for 48 weeks, compared to six percent of patients on placebo (p=0.049). Change in HBV viral load, as measured by the Roche Amplicor® Monitor™ Test (PCR), also was observed. Patients in the adefovir dipivoxil 10 mg arm had a median reduction in HBV DNA from baseline of 3.56 log10 copies/mL, compared to a reduction of 0.55 log10 copies/mL in patients receiving placebo (p<0.001). this="" equates="" to="" a="" 99.97="" percent="" reduction="" in="" circulating="" virus="" in="" patients="" on="" 10="" mg="" treatment.="" additionally,="" following="" 48="" weeks="" of="" treatment="" with="" adefovir="" dipivoxil="" 10="" mg,="" a="" median="" reduction="" in="" alt="" levels="" of="" 49="" iu/l="" was="" observed,="" compared="" to="" a="" reduction="" of="" 17="" iu/l="" in="" the="" placebo="" group=""><0.0001). forty-eight="" percent="" of="" patients="" treated="" with="" adefovir="" dipivoxil="" achieved="" normalization="" of="" alt="" levels,="" compared="" to="" 16="" percent="" of="" patients="" receiving="" placebo=""><>
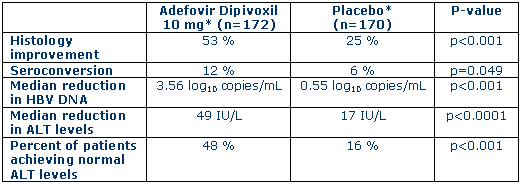
*These data are from an intent-to-treat analysis. Missing values are counted as treatment failures.
"The histology results from this Phase III clinical trial of adefovir dipivoxil 10 mg confirm the antiviral activity observed in our earlier studies and suggest that this compound may offer a meaningful clinical benefit to patients with chronic HBV infection," said John C. Martin, Ph.D., President and CEO, Gilead Sciences. "With positive data from this study and anticipated data from our second Phase III clinical trial, Study 438, later this year, we intend to submit regulatory filings for approval of adefovir dipivoxil 10 mg in early 2002."
Safety Profile
Through the first 48 weeks of Study 437, the discontinuation rate was similar between the treatment and placebo arms, with seven percent of patients receiving adefovir dipivoxil 10 mg and eight percent of patients receiving placebo discontinuing from study. No patients in either the adefovir 10 mg or placebo groups had increases in serum creatinine of greater than or equal to 0.5 mg/dL from baseline or a serum phosphorus level less than 1.5 mg/dL, laboratory markers of renal function, as confirmed by two consecutive laboratory assessments. Additionally, the incidence of grade 3 and 4 laboratory abnormalities and clinical adverse events was similar between the adefovir dipivoxil 10 mg and placebo arms.
Resistance Profile
Preliminary genotypic analyses from Study 437 show that after 48 weeks of treatment, no adefovir resistance mutations were observed. These data confirm Gilead's earlier clinical and in vitro studies, which demonstrated that treatment with adefovir dipivoxil does not lead to the development of resistance.
Adefovir Dipivoxil 30 mg Data
Study 437 included an exploratory arm for the first 48 weeks to evaluate the treatment effect of adefovir dipivoxil 30 mg on seroconversion rates and other measures of efficacy. Data from this third arm indicate similar efficacy results for adefovir dipivoxil 10 and 30 mg in terms of liver histology, seroconversion and reduction in HBV DNA. Mild increases in levels of serum creatinine of greater than or equal to 0.5 mg/dL were observed in nine percent of patients who received 48 weeks of treatment with adefovir dipivoxil 30 mg, as confirmed by two consecutive laboratory assessments. These results are similar to data from Gilead's earlier studies and reaffirm Gilead's strategy to seek regulatory approval only for the 10 mg dose of adefovir dipivoxil.
Adefovir Dipivoxil Phase III Program
In addition to Study 437, a second pivotal Phase III trial is currently underway to evaluate adefovir dipivoxil 10 mg monotherapy as a potential treatment for chronic HBV infection. Study 438 is a randomized, double-blind, placebo-controlled trial which is evaluating adefovir dipivoxil 10 mg for the treatment of hepatitis B "e" antigen negative patients (precore mutant HBV infection). This trial is being conducted in Australia, Canada, France, Greece, Israel, Italy and Southeast Asia. Enrollment was completed in June 2000 with 185 patients. Gilead expects to announce results from Study 438 during the second half of this year and anticipates potential U.S. and European filings for regulatory approval during the first half of 2002.
Adefovir dipivoxil is an investigational compound and has not yet been determined safe or efficacious in humans for its ultimate intended use. Patients and physicians who would like more information about adefovir dipivoxil may contact Gilead Sciences Medical Information at 1-800-GILEAD-5 (1-800-445-3235) or 1-650-574-3000 from outside the United States.
Chronic Hepatitis B Infection
Worldwide, there are approximately 400 million chronic carriers of HBV, of which approximately one million die each year from complications of the disease, making chronic HBV one of the 10 most common causes of death. In the United States, the disease claims between 4,000 and 5,000 lives annually among the 1.25 million Americans infected with chronic HBV. Complications of chronic HBV include cirrhosis, liver failure and primary liver cancer (hepatocellular carcinoma).
Conference Call
Gilead will host a conference call today, June 22, 2001, at 8:30 a.m. (Eastern) to discuss these results. The dial-in numbers for the call are 888-674-1998 (domestic) and 212-231-6047 (international). A replay of this call will be available from 11:00 a.m. June 22, 2001, until 11:00 a.m. June 25, 2001. The replay dial-in numbers are 800-633-8284 (domestic) and 858-587-5842 (international). The password is 19202311.
Gilead Sciences
Gilead Sciences, Inc., headquartered in Foster City, CA, is an independent biopharmaceutical company that seeks to provide accelerated solutions for patients and the people who care for them. Gilead discovers, develops, manufactures and commercializes proprietary therapeutics for challenging infectious diseases (viral, fungal and bacterial infections) and cancer. Gilead maintains research, development or manufacturing facilities in Foster City, CA; Boulder, CO; San Dimas, CA; Cambridge, UK and Dublin, Ireland and sales and marketing organizations in the United States, Europe and Australia.
This press release includes forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, that are subject to risks, uncertainties and other factors that could cause actual results to differ materially from those referred to in the forward-looking statements. Such risks and uncertainties include the risk that the safety, efficacy and resistance data observed in Gilead's earlier clinical trials and preclinical testing may not be observed in Gilead's more reliable Phase III clinical trials, and other risks related to regulatory approval of adefovir dipivoxil. The reader is cautioned not to rely on these forward-looking statements. These and other risks are described in detail in the Gilead Annual Report on Form 10-K for the year ended December 31, 2000 and in Gilead's Quarterly Reports on Form 10-Q, all of which are on file with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Gilead and Gilead assumes no obligation to update any such forward-looking statements.
Other News
Some of the content on this page is not intended for users outside the U.S.
