Share Article
Product Revenues Increased 20 Percent Over Third Quarter 2000
Foster City, CA -- October 25, 2001
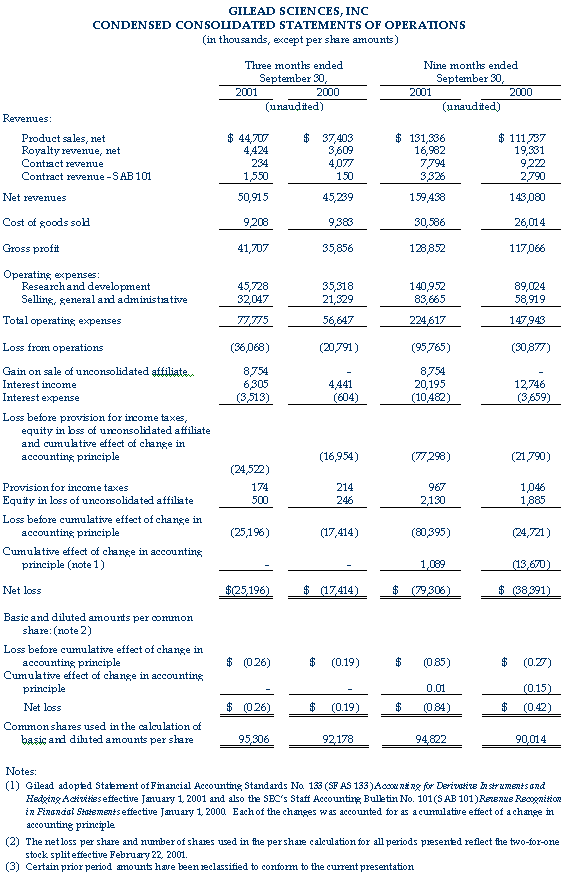
Gilead Sciences, Inc. (Nasdaq: GILD) announced today its results of operations for the third quarter ended September 30, 2001. For the third quarter, Gilead recorded revenues from net product sales of $44.7 million, royalty revenues of $4.4 million and contract revenues of $1.8 million. Total revenues for the third quarter ended September 30, 2001 were $50.9 million, compared to total revenues of $45.2 million for the third quarter of 2000. Revenues for the third quarter of 2000 included net product sales of $37.4 million, royalty revenues of $3.6 million and contract revenues of $4.2 million.
The net loss for the third quarter 2001 was $25.2 million, or $0.26 per share. This compares to a net loss in the third quarter 2000 of $17.4 million, or $0.19 per share.
On August 31, 2001, Gilead sold its 49 percent interest in Proligo L.L.C. ("Proligo") for $14.3 million in cash. Proligo was a joint venture between Gilead and SKW Americas, Inc. focused on the manufacturing of oligonucleotides. SKW Americas, a subsidiary of Degussa Corporation, held the remaining 51 percent of Proligo. The proceeds, net of Gilead's investment in Proligo, are reflected as a gain on the sale of unconsolidated affiliate in the quarter. The recognized gain was $8.8 million, or $0.09 per share.
Net revenues from product sales were primarily derived from sales of AmBisome® (amphotericin B) liposome for injection, accounting for 92 percent of product sales for the third quarter of 2001. AmBisome sales for the third quarter of 2001 were $41.1 million, an increase of 17 percent compared to the third quarter of 2000. Excluding the impact of foreign currencies relative to the U.S. dollar, AmBisome sales grew 20 percent for the third quarter of 2001 over the comparable quarter of 2000. In addition, Gilead recorded other product sales of $3.6 million during the third quarter of 2001, compared to $2.4 million in the third quarter of 2000.
For the third quarter of 2001, royalty and contract revenues resulting from collaborations with corporate partners totaled $6.2 million. These revenues include contract revenues for research and development projects, royalties on sales of AmBisome in the United States by Gilead's co-promotion partner Fujisawa Healthcare, royalties on sales of Tamiflu™ (oseltamivir phosphate) by Hoffmann-La Roche and royalties on product sales of Vistide® (cidofovir injection) outside the United States by Pharmacia Corporation.
Research and development expenses for the third quarter of 2001 were $45.7 million, compared to $35.3 million for the same quarter in 2000. The higher spending during the third quarter of 2001 is attributable to Gilead's expenses associated with the Phase III clinical program for adefovir dipivoxil for hepatitis B virus (HBV) and a $3.0 million clinical milestone payment to Cubist Pharmaceuticals, Inc. ("Cubist") under the European licensing agreement for Cidecin® (daptomycin for injection) signed in January 2001.
Selling, general and administrative expenses for the three months ended September 30, 2001 were $32.0 million, compared to $21.3 million for the same quarter of 2000. The additional spending is primarily due to Gilead's increased global marketing efforts and the expansion of Gilead's U.S. and European sales forces in anticipation of the commercial launch of Viread™ (tenofovir disoproxil fumarate) for HIV.
Net interest income for the third quarter 2001 was $2.8 million, compared to $3.8 million for the same quarter in 2000.
The company also reported equity in the loss of its unconsolidated affiliate of $0.5 million and $0.2 million for the third quarter ended September 30, 2001 and 2000, respectively. The loss for the third quarter was derived from Gilead's 49 percent interest in Proligo prior to August 31, 2001, the date of sale.
Gilead also reported its results of operations for the nine months ended September 30, 2001. The company recorded net revenues from product sales of $131.3 million and aggregate contract and royalty revenues of $28.1 million. Accounting for 93 percent of product sales, AmBisome sales for the nine months ended September 30, 2001 were $121.9 million, a 16 percent increase over the nine months ended September 30, 2000. Excluding the impact of the decline in foreign currencies relative to the U.S. dollar, AmBisome sales grew by 21 percent in the first nine months of 2001 over the comparable period of 2000. Net revenues of $159.4 million in the nine months ended September 30, 2001 compare to net revenues of $143.1 million in the first nine months of 2000. Net revenues for the first nine months of 2000 included product sales of $111.7 million and aggregate contract and royalty revenues of $31.3 million.
The net loss for the nine months ended September 30, 2001, including the cumulative effect of a change in accounting principle related to the company's adoption of Statement of Financial Accounting Standards No. 133, was $79.3 million, or $0.84 per share. This compares to a net loss of $38.4 million, or $0.42 per share for the nine months ended September 30, 2000, including the cumulative effect of the adoption of Staff Accounting Bulletin 101 of $13.7 million, or $0.15 per share.
Research and development expenses for the nine months ended September 30, 2001 and 2000 were $141.0 million and $89.0 million, respectively. The higher spending during the first nine months of 2001 was attributable in part to the recognition of $10.6 million of a $13.0 million up-front payment and $4.2 million of clinical milestone payments to Cubist under the European licensing agreement for Cidecin. In addition, Gilead's expenses associated with the Phase III clinical trials and expanded access programs for Viread for HIV and the Phase III clinical programs for adefovir dipivoxil for HBV increased significantly during the first nine months of 2001.
Selling, general and administrative expenses for the nine months ended September 30, 2001 were $83.7 million compared to $58.9 million for 2000. The additional spending is primarily due to Gilead's increased global marketing efforts and the expansion of Gilead's U.S. and European sales forces in anticipation of the commercial launch of Viread for HIV.
Net interest income for the nine months ended September 30, 2001 was $9.7 million, compared to $9.1 million for 2000.
The company also reported equity in the loss of its unconsolidated affiliate of $2.1 million and $1.9 million for the nine months ended September 30, 2001 and 2000, respectively.
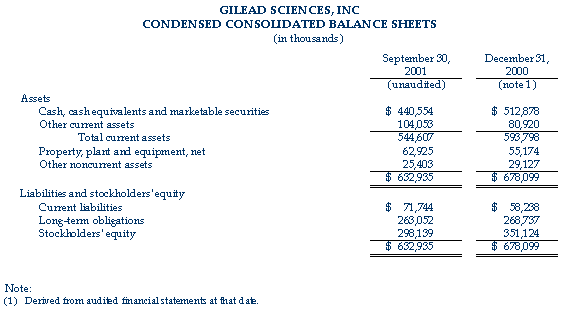
As of September 30, 2001, the company had cash, cash equivalents and marketable securities of $440.6 million, compared to $512.9 million at December 31, 2000.
Corporate Highlights
Today, Gilead announced the appointment of Gayle E. Wilson to the Board of Directors, increasing the Board to eight members. Mrs. Wilson will also serve on the Compensation Committee of the Board of Directors. Mrs. Wilson served as First Lady of California from 1991-1999. Her career has primarily focused on volunteerism, early childhood health and math and science education.
On October 23, Gilead announced that it out-licensed all remaining intellectual property rights under the SELEX(tm) (Systemic Evolution of Ligands through EXponential Enrichment) process patent estate to Archemix Corporation of Cambridge, MA. Gilead has retained a non-exclusive right to utilize this technology for internal research and target validation purposes. Gilead will receive $17.5 million in cash, $9.0 million in 2001 and $8.5 million in 2002. Additionally, Gilead will receive warrants in Archemix.
As stated earlier, in August, Gilead sold its 49 percent interest in Proligo L.L.C. to Degussa Corporation for $14.3 million in cash. Gilead recorded a net gain of $8.8 million from the sale.
Products and Pipeline Highlights
"Our strong third quarter performance reflects our progress toward achieving our financial goals for the year," said John C. Martin, Ph.D., President and Chief Executive Officer, Gilead Sciences. "In addition to the continued solid sales performance from AmBisome, we achieved several key R&D milestones during the quarter, including positive data from our second pivotal Phase III study of adefovir dipivoxil for HBV, as well as the receipt of positive recommendations from both the U.S. Food and Drug Administration's (FDA) Antiviral Drugs Advisory Committee and the European Union's Committee for Proprietary Medicinal Products (CPMP) in support of the use of Viread in HIV-infected patients. These significant milestones augment our momentum towards becoming a worldwide leader in advancing treatments for challenging human diseases."
Viread™ (tenofovir disoproxil fumarate) for HIV
Last week, Gilead announced that the CPMP recommended granting of the Marketing Authorisation for Viread in the European Union's 15 member states. On the basis of the safety and efficacy data submitted for Viread, the committee has recommended the granting of a Marketing Authorisation under exceptional circumstances. The indication recommended by the CPMP is for Viread taken in combination with other antiretroviral agents in HIV-infected patients over 18 years of age experiencing early virological failure. Gilead anticipates the European Medicines Evaluation Agency's authorization will be granted in early 2002.
In October, Gilead received unanimous support from the FDA's Antiviral Drugs Advisory Committee for the use of Viread in the treatment of HIV infection in adult patients who have received prior antiretroviral therapy. The committee found that the risk/benefit ratio of Viread was favorable for patients in the principal clinical studies. The FDA will consider the committee's comments as it completes its priority review of the Viread New Drug Application. Gilead anticipates an action by the FDA by November 1, 2001. To further demonstrate the efficacy and safety of Viread in a broader patient population, Gilead will complete an ongoing confirmatory 96-week, 601-patient Phase III clinical trial (Study 903) in treatment-naive patients with HIV infection.
Adefovir Dipivoxil for Chronic HBV Infection
Also in September, Gilead announced results from the second of two pivotal Phase III studies, Study 438, which evaluated the safety and efficacy of adefovir dipivoxil at 10 mg as a once daily monotherapy compared to placebo in patients infected with precore mutant chronic HBV. The study met the primary endpoint of improvement in liver histology, as well as the secondary efficacy endpoints. Gilead intends to submit regulatory filings for approval of adefovir dipivoxil in both the United States and Europe in the first half of 2002.
Cidecin® (daptomycin for injection) for the Treatment of Gram-positive Bacterial Infections Earlier this month, Cubist announced preliminary results from Study 9801, a Phase III safety and efficacy study of Cidecin for the treatment of complicated skin and soft tissue infections resulting from Gram-positive bacteria. The data will be presented this Friday, October 26, at the 39th Annual Meeting of the Infectious Disease Society of America taking place in San Francisco. Cubist anticipates filing for regulatory approval of Cidecin in the United States mid-2002, and Gilead expects to follow with a filing in Europe. Gilead holds the exclusive rights to commercialize and market Cidecin in Europe.
Conference Call
Gilead will host a conference call today, October 25, 2001, at 4:30 p.m. Eastern. To access the live call or the seven-day archive via the internet, log on to www.gilead.com. Please connect to the company's website at least 15 minutes prior to the conference call to ensure adequate time for any software download that may be needed to hear the webcast. Alternatively, please call 888-489-9485 (U.S.) or 212-676-5065 (international). Telephone replay is available approximately one hour after the call through 7:00 p.m. EDT, October 28, 2001. To access, please call 800-633-8284 (U.S.) or 858-812-6440 (international). The conference ID number is 19878561. The information provided on the teleconference and on the webcast is only accurate at the time of the call, and Gilead will take no responsibility for providing updated information.
About Gilead
Gilead Sciences, Inc., headquartered in Foster City, CA, is an independent biopharmaceutical company that seeks to provide accelerated solutions for patients and the people who care for them. Gilead discovers, develops, manufactures and commercializes proprietary therapeutics for challenging infectious diseases (viral, fungal and bacterial infections) and cancer. Gilead maintains research, development or manufacturing facilities, and sales and marketing organizations in the United States, Europe and Australia.
Forward-looking Statements
Statements included in this press release that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include those regarding Gilead's future financial results, including: revenues, research and development expenses, selling, general and administrative expenses, the safety and efficacy of any marketed or pipeline development products, the ability to file for or obtain marketing approval for Gilead's pipeline development products, the uses for which our pipeline development products may be approved and the competitive positioning of our marketed or pipeline development products. Gilead cautions readers that forward-looking statements are subject to certain risks and uncertainties, which could cause actual results to differ materially. These risks and uncertainties include those that can cause fluctuations in our financial results, such as our ability and the ability of our partners to successfully market our products and maintain or grow market share particularly in light of new competitive products being launched by our competition; our ability to predict the timing and amount of spending in our research and clinical programs; fluctuations in foreign currency against the U.S. dollar; the ability of our partners to achieve and the timing of milestones including those requiring payments from Gilead to the partner and those requiring payments from the partner to Gilead, as well as risks and uncertainties that affect our future prospects such as the risk that we may not continue to observe the safety, tolerability and efficacy data for our products and product candidates that we are observing today; other risks relating to the regulatory approval of our products and product candidates; and other risks identified from time to time in the company's reports filed with the U.S. Securities and Exchange Commission. The company directs readers to its Annual Report on Form 10-K, for the year ended December 31, 2000, filed in March 2001, and its 2001 Quarterly Reports on Form 10-Q filed with the SEC. Gilead claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements.
###
AmBisome and Vistide are registered trademarks, and Viread is a trademark of Gilead Sciences, Inc.
Tamiflu is a trademark of F. Hoffmann-La Roche Ltd.
Cidecin is a registered trademark of Cubist Pharmaceuticals, Inc.
Other News
Some of the content on this page is not intended for users outside the U.S.
