Share Article
When Jan Pineda was a child, she saw firsthand the terrible effects cancer had on people she loved. That was why she decided to pursue a career in science – to make a difference for patients with cancer and their families – ultimately becoming a cell therapy specialist at Kite.
It’s also one of the things that motivates her each time she steps into the manufacturing facility at Kite’s site in El Segundo, California.
“The patients are my biggest motivation. Our work is very specific to each patient – we are literally holding a person’s cells in our hands,” says Jan. “The responsibility of this is enormous, and I’m honored to be a part of a team that does this work.”
Jan joined Kite nearly three years ago, immediately following the approval of the company’s first chimeric antigen receptor (CAR T) cell therapy for the treatment of certain types of lymphoma. She quickly made the transition from a college student to being at the forefront of manufacturing potentially life-saving therapies for people living with blood cancer.
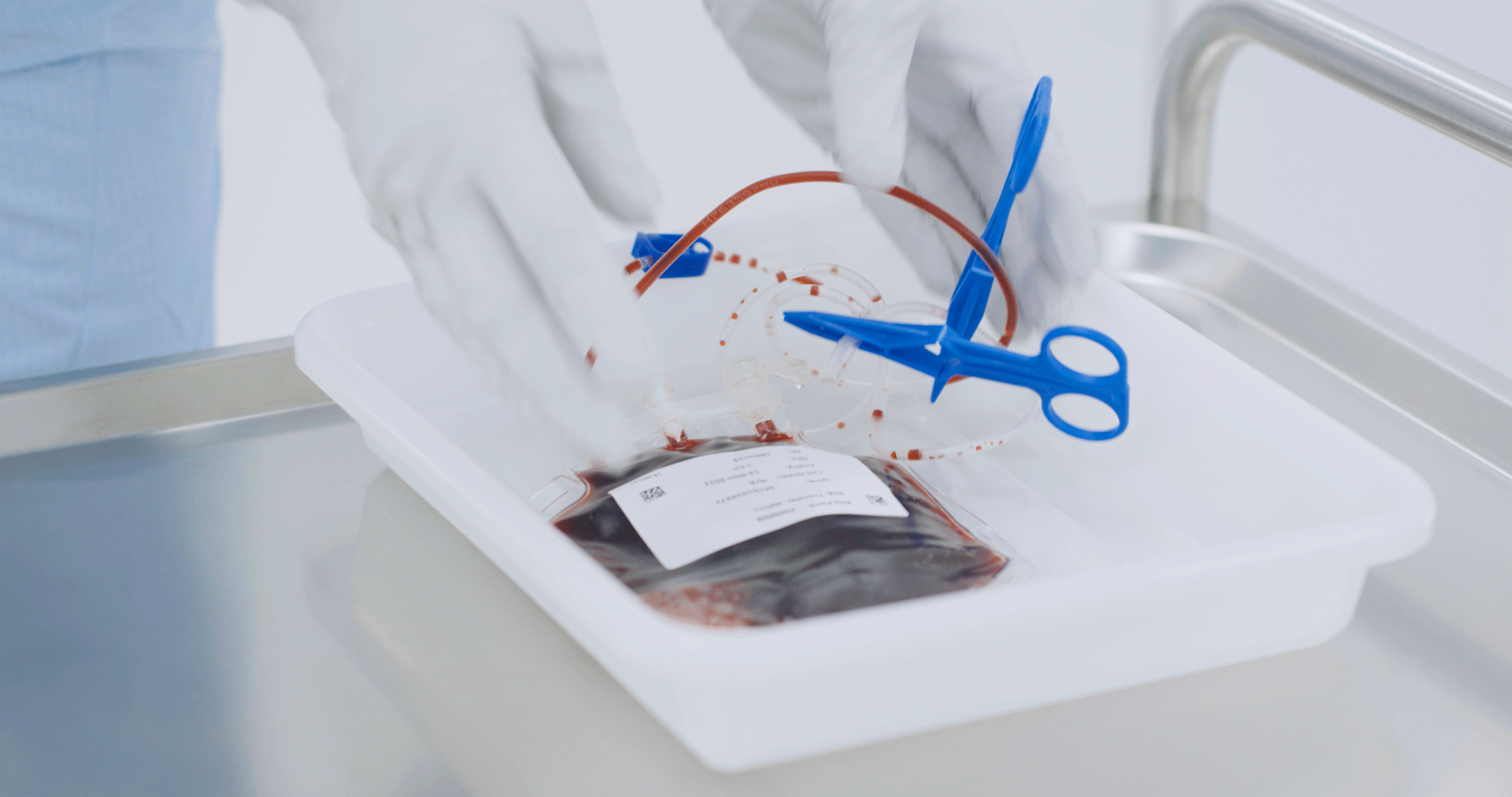
CAR T therapy is a kind of personalized medicine that involves engineering a patient’s own white blood cells to recognize and attack cancer cells. The multi-step process begins when a patient has blood drawn and the white blood cells are separated out in a process called leukapheresis. Those cells are then flown to El Segundo, where specialists such as Jan engineer the cells to fight cancer. Once the cells are received, the T cells are isolated and engineered with the CAR gene. The cells are then multiplied, frozen and shipped back to the center where the patients are being treated.
“When people ask me what I do for a living, I tell them, that in a way, I help train patients’ cells to fight cancer,” says Jan.
The CAR T manufacturing process is complex and time-sensitive. Patients who are eligible for CAR T therapy often have exhausted other treatment options, face an otherwise difficult prognosis and have the potential for rapid disease progression. It is critical that the process happens swiftly so that the treatment gets to the person as quickly as possible – in the United States, the entire process from leukapheresis to product release takes a median of 16 days.
However, the need to move quickly must also be balanced with incredible precision and extreme care when handling someone’s cells. As a cell therapy specialist, Jan plays a particularly important role in this part of the process. She visually inspects the product bag, a container holding the cells, over a white and black background. She looks for any defects such as leaks or unwanted particles, and ensures that the color and clarity of the batch of cells meet established standards. If a product bag does not pass this inspection, then a new one must be created.
“It’s my job to ensure the product bag meets our quality standards before it is frozen and shipped back for patient reinfusion,” says Jan. “Very few people have this responsibility, so it’s a rewarding position.”
Jan says it’s often assumed that manufacturing is not a very patient-centric role. But when she’s holding the cells, she thinks about how important the process is for the person who is awaiting treatment.
“Each move our team makes can have a direct effect on the life of someone else. It keeps you focused because you’re always thinking about your decisions at each step: ‘Is this the best I can do? What if these were the cells of someone I know and love?’” says Jan. “We’re working to help people living with cancer. It’s hard to imagine a more purposeful career path than that.”